Lattice oxygen insertion mechanism in CeO2 catalyzed reactions in water: nitrile hydration reaction
Abstract
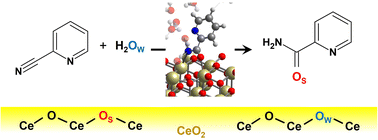
Cerium oxide (CeO$_2$) exhibits prominent catalytic activity in various organic reactions owing to its unique acid–base and redox properties. One of the most interesting applications of pure CeO$_2$-catalyzed organic reactions is the hydration of nitriles in water. The experimental results showed that the hydration of 2-cyanopyridine to picolinamide in water using CeO$_2$ catalysts proceeds readily at low temperatures (30–100 °C) in high yields and that this reaction occurs exclusively on CeO$_2$ among various metal-oxide catalysts. To elucidate the unique catalytic activity of CeO$_2$, the reaction mechanism is dissected using the density functional theory-based molecular dynamics (DFT-MD) simulations. Based on the free energy analysis, it is demonstrated that the reaction proceeds with the involvement of the surface lattice oxygen, where the lattice oxygen atom is inserted into picolinamide. The involvement of the surface lattice oxygen is notably uncommon given the low temperatures of the reaction, and this computational prediction is verified by the two experiments using H$_2$ ${}^{18}$O solvent and $^{18}$O-exchanged CeO$_2$ catalyst, where the introduction of surface lattice oxygen into picolinamide is confirmed. The inherent flexibility of the surface lattice oxygen and the unique acid–base properties of CeO$_2$, which can favorably bind and activate both nitrile and water molecules, are key factors in the high reactivity for various organic reactions, which characterizes the outstanding catalytic activity of CeO$_2$.